electron configuration of mg2+|Mg 2+ Electron Configuration (Magnesium Ion) : Clark The electron configuration of Magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of Magnesium is . iWank.TV - 15+ millon Free Porn videos from most popular tube sites: Française, Mamie, Mature, Transexuelle, Lesbiennes, Echangistes, Maman, Maman Salope, Ados .
PH0 · What is the electronic configuration of Mg^(2+) ion?
PH1 · Mg 2+ Electron Configuration (Magnesium Ion)
PH2 · MG2+ Lewis Structure : Drawings, Hybridization, Shape,
PH3 · How to Write the Electron Configuration for Magnesium (Mg)
PH4 · Electron configuration of magnesium
PH5 · Electron Configuration of Ions
PH6 · Electron Configuration for Magnesium (Mg, Mg2+ ion)
PH7 · Electron Configuration for Magnesium (Mg, Mg2+ ion)
PH8 · Electron Configuration Calculator
PH9 · 7.4: Electron Configurations of Ions
The WordReference English-German Dictionary is a living, growing dictionary. It contains over 66775 terms and 251036 translations in both English and German, and it will continue to grow and improve. Thousands more terms that are not included in the main dictionary can be found in the WordReference English-German forum questions and answers.
electron configuration of mg2+*******Wayne Breslyn. 757K subscribers. Subscribed. 925. 138K views 4 years ago. In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We’ll also look at .
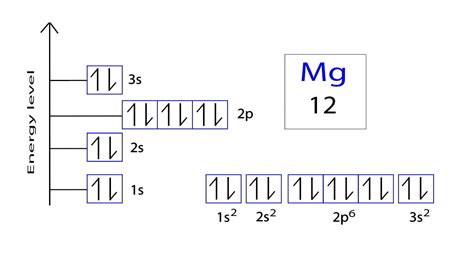
The arrangement of electrons in magnesium in specific rules in different orbits and orbitals is called the electron configuration of magnesium. The electron configuration of magnesium is [ Ne] 3s 2 , if .
The electron configuration of the magnesium ion Mg2+ is similar to Na+, Ne, and Al3+. It is not similar to Ar. Okay, so we are given these answer choices, but how do we know which 3 share the same .electron configuration of mg2+The electron configuration of Magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of Magnesium is . © 2024 Google LLC. This chemistry video tutorial explains how to find the electron configuration of Ions such as Mg2+, P3-, Fe2+, and Fe3+.Electron Configuration - Basic Intro:. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy. Ground state means that the atom has the lowest energy .In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence shell, the configuration of magnesium is represented as follows: Mg → 1s2 2s2 . The electronic configuration of Mg can be shown as 1s 2 2s 2 2p 6 3s 2 and that of Mg 2+ is found to be 1s 2 2s 2 2p 6. The electron distribution among various .In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in .
Electronic Configurations of Cations and Anions. Paramagnetism. Diamagnetism. Practice. How to tell if a substance is paramagnetic or diamagnetic. Contributors. Learning .
Step 1. Atomic number of Mg is 12. Write the electron configuration for Mg2+ ion. Select the correct answer below: Mg?! : 1822822p! Mg?! : 1:22:26 Mg?! : 18228273s?
Electronic Configuration. The electronic configuration of the element gives the information about the distribution of electrons in the different orbitals. 2 electron can be filled in one orbital having opposite spin. s-subshell can have 2 electrons, p-subshell can have 6 electrons and so on. Answer and Explanation: 1The electrons are filled in the orbitals according to the Aufbau principle. It states that filling of electrons takes place in increasing order of energy. The energy increases with increasing value of n + l. O r b i t a l 1 s 2 s 2 p (n + l) v a l u e 1 2 3 Since, 10 electrons are present in magnesium ion, the electronic configuration is given .What is the correct electron configuration of a magnesium ion, Mg2+ ? A. [Ne] 3s 1 B. [Ne] C. [Ar] D. [Ar] 3s 1 E. [Ne] 3s . The correct option is B. Magnesium (Mg) is an alkaline earth metal having an atomic number of 12. Thereby, the electronic configuration of magnesium is [Ne]3s². When Mg losses two. Continue reading. Discover more from: .Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells.
To write the electron configuration for Mg2+, we start with the electron configuration of neutral magnesium: 1s 2 2s 2 2p 6 3s 2. Then, we remove two electrons from the highest energy level, which is the 3s orbital. Thus, the electron .Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .Mg 2+ Electron Configuration (Magnesium Ion) In the fluorine ground-state electron configuration, the five electrons of the 3p orbital are located in the p x, p y, and p z orbitals. Then correct electron configuration of fluorine in the ground state will be 1s 2 2s 2 2p x 2 2p y 2 2p z 1. This electron configuration shows that the last shell of the fluorine atom has an unpaired electron . The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

镁的电子构型. 镁的电子构型是 [Ne] 3s 2. 化学元素镁以其原子序数 12、符号 Mg 和原子质量 24.305 u 为特征。. 它被认为是在地壳中发现的所有元素中的第七种,是盐和氧化物等各种化合物的一部分,总是不溶的。. 同时,它是海洋中的第三个。. 虽然它是地球上最 .The electronic configuration of a Mg atom is 2, 8, 2 and has 12 electrons. Mg2 has a positive charge, which means two electrons are lost, and the number of electrons left in the atom is 12 to 2=10. As a result, in Mg2+, there are .electron configuration of mg2+ Mg 2+ Electron Configuration (Magnesium Ion) The electronic configuration of Mg 2+ is. Chemistry. JAMB 2012. The electronic configuration of Mg 2+ is A. ls 2 2s 2 2P 6 3s 2 3P 2; B. Is 2 2s 2 2P 6; C. ls 2 2s 2 2p 6 3s 2; D. ls 2 2s 2 2P 4; Correct Answer: Option B Explanation No official explanation is available for this question at this time. Please check contributions posted by others . The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.
Mg → 1s2 2s2 2p6 3s2 Mg2+ → 1s2 2s2 2p6. Cela indique que : Il y a 2 électrons dans l'orbitale 1s. . Dans ce cas il correspond au néon avec la configuration Electron : 1s2 2s2 2p6. Puisque ses premiers niveaux d'énergie correspondent à ceux du magnésium, c'est-à-dire que les premières orbitales sont les mêmes dans le magnésium et .
The electron configuration of magnesium (Mg) in its neutral state is 1s2 2s2 2p6 3s2. When magnesium loses two electrons to form a magnesium ion with a 2+ charge (Mg2+), its electron configuration becomes 1s2 2s2. This means that the 3s2 subshell is now empty, and the magnesium ion has a full outer shell with a total of two electrons.The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 2.6.2 2.6. 2 ): A superscript number that designates the number of electrons in that particular subshell. The electron configuration for Mg2+ is 1s22p22p6. What is the electronic configuration of the atom C6? what is the electronic configuration of the atomC6. How many electrons are in an Mg2 plus ion?
Win draw win predictions require a lot of data to be verified and, frankly, it’s a very time-consuming activity. To spare yourself from the gruelling research, bet on BTTS markets. A significant reason for the popularity of this market is its non-reversible nature. The moment the second net is grazed, you can stop caring and relax.
electron configuration of mg2+|Mg 2+ Electron Configuration (Magnesium Ion)